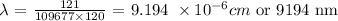Answer:
Step-by-step explanation:
Here, we want to get the wavelength of light emitted when an electron transition from n = 11 to n = 1 state
What this means is that it is going from a higher energy level to a lower energy level
What happens with this type of movement is that energy is released by the transiting atom
To get the wavelength of this, we use the Rydberg equation as follows:
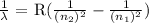
where lambda is the wavelength
R is the Rydberg's constant which is 109,677 cm^-1
n2 is the final state which is 1
n1 is the initial state which is 11
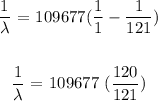
We have this as: