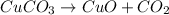According to the given reaction, the reaction is a decomposition reaction, this is because a substance (CuCO₃) is broken down to simpler molecules.
To balance it we have to pay attention to subscripts and coefficients that indicate the number of atoms and molecules respectively.
For example, in CuCO3, there is one atom of copper (reactants) and in CuO there is also one atom of copper (products). It means that the copper is already balanced.
There is one atom of carbon in the reactants and one of them in the products, the carbon is also balanced.
There are 3 atoms of oxygen in the reactants and 1+2 of them in the products (CuO and CO₂). The oxygen is balanced too.
It means that the equation is already balanced and the coefficients of all molecules should be 1, but remember this is redundant so you just have to leave the equation just as it is.