Answer:
0.55g of MgO will be formed.
Step-by-step explanation:
1st) From the balanced equation we know that with 2 moles of Mg, 2 moles of MgO are formed. It is necessary to use the molar mass of Mg and MgO to convert moles to grams:
- Mg molar mass: 24.3g/mol
- Mg conversion:
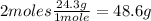
- MgO molar mass: 40.3g/mol
- MgO conversion:
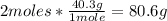
Now we know that with 48.6g og Mg, 80.6g of MgO are formed.
2nd) Finally, with the calculated grams from the stoichiometry of the reaction, and the starting mass of Mg (0.33g) we can calculate the grams of MgO that will be formed:
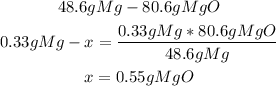
So, 0.55g of MgO will be formed.