Answer:
2694.56grams.
Explanations
Given the balanced chemical reaction between sulphuric acid and ammonia expressed as:
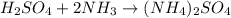
Given the following parameter
moles of ammonia = 58.82 moles
Mass of sulphuric acid = 2kg = 2000grams
Determine the moles of H2SO4
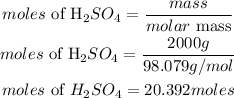
Since the moles of sulphuric acid is less than the moles of one atom of ammonia, hence sulphuric acid will be the limiting reactant.
Determine the maximum mass of ammonium sulphate
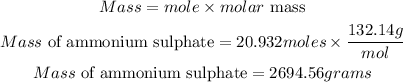
Hence the mass of ammonium sulphate produced is approximately 2694.56grams