Answer:
1.19 x 10²¹ atoms of Cu (copper).
Step-by-step explanation:
What is given?
Mass of copper (Cu) = 0.125 g.
Molar mass of Cu = 63.5 g/mol.
Avogadro's number = 6.022 x 10²³ atoms/mol.
Step-by-step solution:
First, let's convert 0.125 g of Cu to moles using its molar mass, like this:
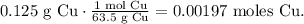
And now, let's use the Avogadro's number, which is telling us that there are 6.022 x 10²³ atoms of a certain element in 1 mol. The conversion will look like this:
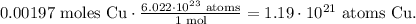
The answer is that we have 1.19 x 10²¹ atoms of Cu (copper) in 0.125 g of Cu (copper).