Answer: the best option to answer the question is letter A ("B) NaCl(aq) and MgCO3(s)")
Step-by-step explanation:
The question requires us to determine the products of the reaction between MgCl2 and Na2CO3, which occurs through a double replacement mechanism.
A double replacement (or double displacement) reaction can be described by the following generic chemical equation:
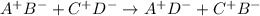
where A and C are cations, while B and D are anions, and a displacement of both B and D occurs forming two new compounds.
To identify the products of the reaction between MgCl2 and Na2CO3, first we need to identify the cations and anions in these compounds and their charges:
- Mg is a metal that forms a cation with charge +2 (Mg2+);
- Cl usually forms an anion with charge -1. Also, the combination of 2 Cl atoms with 1 Mg atom indicates that the charge of the anion is -1;
- Na is a metal that forms a cation with charge +1 (Na+);
- CO3 is an anion with charge -2;
Considering the information above, we can write the double replacement reaction between MgCl2 and Na2CO3 as:
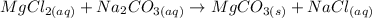
Comparing this reaction with the generic equation written above, we can say that Mg2+ would be the cation A and Na+ would be the cation C, while Cl- would be the anion B and (CO3)- would be the anion D:
A = Mg2+
B = Cl-
C = Na+
D = (CO3)2-
AB = MgCl2
CD = Na2CO3
Therefore, the products formed (AD and CB) would be:
AD = MgCO3
CB = NaCl
Therefore, the best option to answer the question is letter A ("B) NaCl(aq) and MgCO3(s)").