Step 1
Sr(OH)2 is a strong base. It means that it is completely dissociated in an aqueous solution.
Sr(OH)2 => Sr+2 + 2OH-
--------------------------
Step 2
pH is asked here, but there is a base, so let's calculate pOH first.
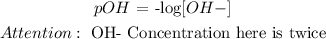
Sr(OH)2 => Sr+2 + 2OH-
0.0538 M 0.0538 M 2 x 0.0538 M
Therefore, pOH = -log (2x0.0538M) = 0.97 approx.
---------------------------
Step 3
Now, pH + pOH = 14 => pH = 14 - pOH = 14 - 0.97 = 13.03
Answer: pH = 13.03