Answer:
Volume, pressure and temperature.
Step-by-step explanation:
In order to determine the number of moles of a gas sample, we need to collect information about the volume, pressure and temperature of the gas, because we can replace those values in the Ideal Gases law formula, and calculate the number of moles:
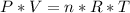
In that formula, we will use the constant of the ideal gases R (0.082 atm.L/mol.K), so we have to make sure that all the collected information has the units atm, liters and Kelvin.