Answer: the concentration of the diluted solution is 0.125 mol/L.
Step-by-step explanation:
The question requires us to calculate the final concentration of a diluted solution prepared by adding 25 mL of water to 125 mL of a 0.15 mol/L NaOH solution.
In a dilution process, the number of moles of a substance does not change, only the volume in which this amount of moles is. Thus, we can write:
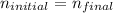
Considering the definition of molarity (also called molar concentration), we can write the following equation to determine the number of moles of a substance:
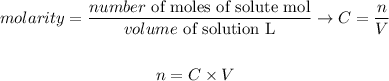
Therefore, we can write the first expression as:
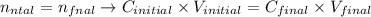
Rearranging this expression, we can calculate the final concentration (Cfinal) that is required by the question.
Remember that the question provided us with the concentration of the initial solution (Cinitial = 0.15 mol/L), the volume taken from the initial solution (Vinitial = 125 mL) and we can calculate the volume of the final solution as a sum of the Vinitial and the volume of water used (Vfinal = 25 + 125 = 150 mL).
Therefore, applying these values to the equation above, we'll have:
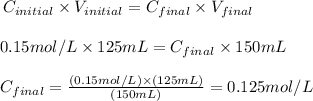
Therefore, the concentration of the diluted solution is 0.125 mol/L.