Answer:
0.159 L.
Step-by-step explanation:
Remember that the formula of molarity is the following:
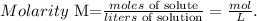
As we have the concentration (molarity) of the stock solution (0.753 M), and the number of moles present in the diluted solution (0.120 mol), we just have to solve for 'liters of solution' and replace the given data:
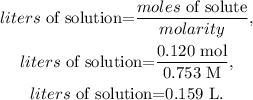
The answer would be that the volume is 0.159 L.