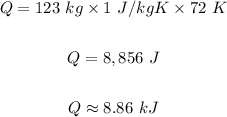Answer
D. 8.86 kJ
Step-by-step explanation
Given that:
Mass of water, m = 123.00 kg
Initial temperature, T₁ = 25 °C = (25 °C + 273) = 298 K
Final temperature, T₂ = 97 °C = (97 °C + 273) = 370 K
Specific heat of water, c = 1 J/kg K
What to find:
The amount of energy (Q) required to cause the temperature rise of the mass of water.
Step-by-step solution:
The amount of energy (Q) required to cause the temperature rise of the mass of water can be calculated using the formula below:
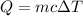
Note that ΔT = T₂ - T₁ = 370 K - 298 K = 72 K
Therefore, the energy require will be: