ANSWER
The final volume of the car tire is 8.9L
Step-by-step explanation
Given information
The initial volume of a car tire is 9.5L
The initial temperature of the car tire is 25 degrees Celcius
The final temperature of the car tire is 5.0 degrees Celcius
To find the final volume of the car tire, follow the steps below
From the given information, you will observe that the pressure of the tire remains constant, so the law governing the situation is called the Charle's law
Step 1: State Charle's law
Charle's law state that the volume of a given is directly proportional to the temperature of the mass, provided that the pressure remains constant.
The above law can be expressed mathematically below
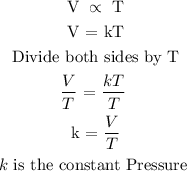
Step 2: Convert the temperature to kelvin from degrees Celcius
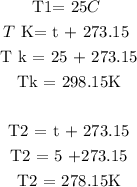
Step 3: Substitute the given data into the formula in step 1
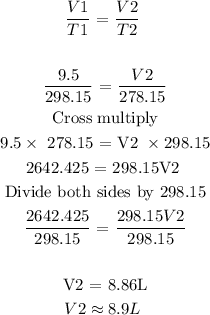
Hence, the final volume of the car tire is 8.9L