1.2x10^2 g of AlCl3 will be produced.
1st) It necessary to calculate the grams of chlorine (Cl2) and aluminum chloride (AlCl3) that are related on the given equation. Here we need to use the molar mass of each compound:
- Cl2 molar mass: 70.9 g/mol
- AlCl3 molar mass: 133 g/mol
The equation shows that with 3 moles of Cl2 we can produce 2 moles of AlCl3. With a mathematical Rule of Three and the molar mass of each compound we can calculate the grams that are needed by formula:
- Chlorine:
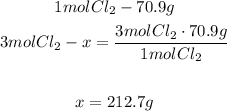
- Aluminum chloride:
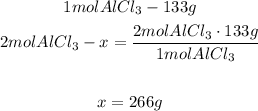
2nd) We can calculate the grams of AlCl3 that will be produced from 92g of Cl2:
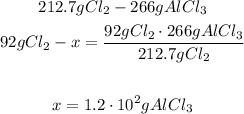
So, 1.2x10^2 g of AlCl3 will be produced.