Answer
: 0.13 moles of KClExplanation:
The balanced chemical reaction :
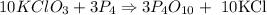
GIVEN
• Mass of Potassium chlorate KClO3 = 16 g
,
• Molecular mass KClO3 = 122,55 g/mol
(1) Calculate moles of KClO3
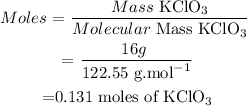
(2) By stoichiometry, the balanced equation informs us that :
10 moles KClO3 produces 10 moles KCl
therefore ;
0.131 moles of KClO3 will produce 0.13 moles of KCl
{ take note that the ratio if 1:1 , meaning for every 1 mole of KClO3 , 1 mole of KCL willbe produced }