The first step to answer this question is to convert the volume of solution needed, that is in liters, to mililiters.
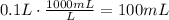
Use the concentration of the solution to find how many mg are present in 100mL of the solution:
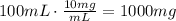
Now, convert the amount of mg present in the solution to grams:
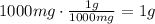
It means that 1g of NaOH is needed to prepare 0.1L of a 10mg/mL solution.