Given the half life of the sample is 12.4 hrs.
Therefore, number of half life in 62.0 hrs is given as,
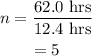
The number of particle left after squence of half life is given as,
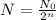
Here,
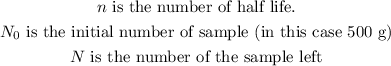
Substituting all known values,
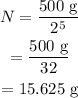
Therefore, after 62.0 hrs 15.625 g of the sample will be left over.