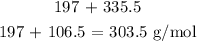Answer:
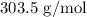
Step-by-step explanation:
Here, we want to get the mass of one mole of AuCl3
This refers to the molar mass of AuCl3
To get this, we have to use the masses of the constituent atoms
The atomic mass of gold is 197 amu
The atomic mass of chlorine is 35.5 amu
Thus, the mass of one mole AuCl3 will be: