Reactions according to Bronsted are those in which there is an exchange of H+ ions. The Bronsted acid will be the substance that donates H+ ions and the base will be the compound that receives them.
As a result we will have a conjugated acid and a conjugated base. The conjugate acid will be the former base that just received the H+ ions. The conjugate base will be the former acid that donated its H+ ions.
Now let's look at the reaction. On the reactants side the compound that can donate H+ ions will be HSO3- and the compound CN- will be the one that receives them. Therefore, HSO3 will be the Bronsted acid (A) and CN will be the base (B).
HSO3 ---> A
CN -----> B
Now let's look at the product side. The compound that received the H+ ions will be the compound HCN, so this will be the conjugate acid (Ac). And the compound that lost its H+ ions is SO3, so it will be the conjugate base (Bc).
HCN --->Ac
SO3 ---->Bc
Comparing the analysis that we did with the reaction we will have that:
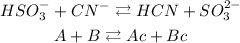
So, the answer will be option number 5