In order to answer question 4 we need to calculate how much 2.56x1024 molecules of water weight.
We know that 1 mol of water molecules (6.022x1023 molecules) weight 18g. This is because according to the periodic table 1 mol of hydrogen weights 1g and 1 mol of oxigen weights 16g. So knowing the chemical formula H2O we calculate:
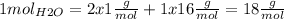
Now we need to calculate how much 2.56x1024 molecules weight (mH2O):
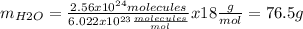
So we know that the mass of water is 76.5 g.
Now we calculate the total weight, given that the cup weights 5g:
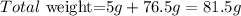
So the correct answer is B: 81.6g