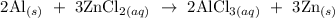ANSWER
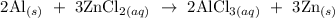
Step-by-step explanation
Given that;
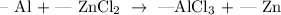
Follow the steps below to balance the above equation
Apply the law of conservation of mass
The law of conservation of mass states that matter can neither be created nor destroyed but can be transform from one form to another. This implies that the number of each atoms of the elements on the reactant side must be equal to the number atom of the elements on the products side.
On the reactants side
1 atom of aluminum
1 atom of zinc
2 atoms of chlorine
On the product sides
1 atom of aluminum
3 atoms of chlorine
1 atom of zinc
To balance the above reaction, 2 moles of Al must react with 3 moles of ZnCl2 to give 2 moles AlCl3 and 3 moles of Zn