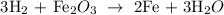Answer:
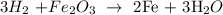
Step-by-step explanation:
Here, we want to balance the given equation
By balancing the chemical equation, it means that the number of atoms of each of the elements is present on both sides of the equation
We start by balancing the number of oxygen atoms. We simply place 3 behind the water molecule, then 2 behind the iron, and 3 behind the oxygen
We have that as: