Answer:
The actual yield is 268g.
Step-by-step explanation:
1st) It is necessary to balance the chemical reaction:
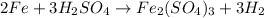
From the balanced reaction we know that 1 moles of Fe2(SO4)3 is formed from 2 moles of Fe and 3 moles of H2SO4.
2nd) With the molar mass of Fe2(SO4)3, we have convert the mole to grams:
- Fe2(SO4)3 molar mass: 400g/mol
So, 1 mole of Fe2(SO4)3 is equal to 400g. This will be the Theroretical yield.
3rd) Now, with the Theoretical yield and the Percent yield, we can calculate the Actual yield of Fe(SO4)3:

Finally, the actual yield is 268g.