We will firstly convert the mass into moles to dfeine the relationship using mole ratio.
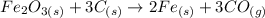
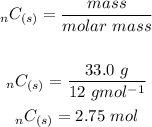
The mole ratio between C and CO is 3:3 or 1:1. Therefore 2.75 mol of carbon would produce 2.75 mol of carbon monoxide. Using this relationship we will convert moles to mass.
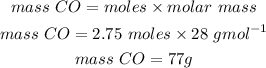
Answer: 77.0g of CO are produced when 33.0g of C reacts.