The function T(x) gives us the time required to reach a coma and x is the amount of carbon monoxide.
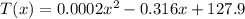
a) Set x=550 and solve for T as follows:
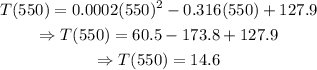
And remember that T is given in hours, then the answer to part a) is 14.6 hr.
b) Set T(x)=6 and solve for x as follows:
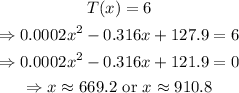
You can solve the quadratic equation by any method you want.
Remember that T(x) requires that 500<=x<=800, we cannot know the effect that 910.8 ppm can cause in a person. Then, the answer to part b) is x=669.2 ppm