Answer 1 : The synthesis reaction is,

Explanation :
Synthesis reaction : It is a type of reaction in which the reactants present in elemental state react to give a single product.
(1)

This is a synthesis reaction.
(2)

This is a single displacement reaction in which the most reactive element displaces the least reactive element.
(3)

This is a decomposition reaction in which larger reactant decomposes to give two or more products.
(4)

This is a neutralization reaction in which an acid and a base react to give a salt and water as a product.
Answer 2 : The number of half-lives it take will be, 3
Formula used :
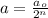
where,
a = amount of reactant left after n-half lives =

 = Initial amount of the reactant
= Initial amount of the reactant
n = number of half lives
Putting values in above equation, we get:


n = 3
Therefore, the number of half-lives it take will be, 3