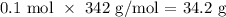Answer:
Step-by-step explanation:
We want to get the mass of the powdered drink
Mathematically:
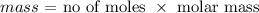
To get the number of moles, we multiply the molarity and the volume
We have the molarity as 1 M
The volume in L would be (100/1000 = 0.1 L)
We have the number of moles as:
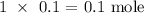
To get the mass, we need the molar mass
The molar mass of sucrose is 342g/mol
Thus, we have the mass as: