Answer
Mass of N₂H₄ = 3.605085 grams
Step-by-step explanation
Given:
Molarity of H₂SO₄ = 0.225 M
Volume of H₂SO₄ = 250 mL = 0.250 L
Equation:
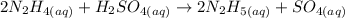
What to find:
The mass of hydrazine, N₂H₄.
Step-by-step solution:
To find the mass of hydrazine first we need to find the moles of hydrazine from the moles of sulfuric acid using given data and given balanced equation above.
Moles of H₂SO₄ in 0.225 M with 250 mL is calculated as follows:
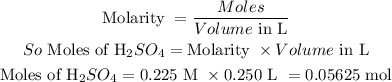
Now we can determine the moles of hydrazine by comparing the moles with the balanced equation for the reaction.
From the balanced equation; 1 mole of H₂SO₄ reacts with 2 moles of N₂H₄,
So 0.05625 mole of H₂SO₄ will reacts with:
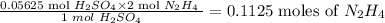
Now using Number of moles = Mass/molar mass, we can then calculate the mass of hydrazine as follows:
From the Periodic Table:
Molar mass of hydrazine = 32.0452 g/mol
Hence, Mass of hydrazine = Number of moles of hydrazine x Molar mass of hydrazine.
Mass of N₂H₄ = 0.1125 mol x 32.0452 g/mol
Mass of N₂H₄ = 3.605085 grams