Step-by-step explanation:
It is known that a general equation for a weak acid is:
HA (aq) + H2O (l) <=> H3O+(aq) + A-(aq)
-----------
At some point, it is needed to find molecules which represent: HA, H3O+, and A- .
After that, it is necessary to calculate the Ka of the acid (but as an approximation) as follows:
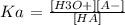
------------
Procedure: (don't forget to count the molecules or ions, it will give us an approximation of the concentration of each)
The bigger the Ka, the stronger the acid. If there is no HA, the denominator would be 0, so it is a strong acid.
-------
Solution 1)
Number of H3O+ = 2; HA = 2, and A- = 2
(we don't consider H2O molecules)
So, Ka = 2 x 2/2 = 2
-------
Solution 2)
Number of H3O+ = 4; HA = 0, and A- = 4
Denominator = 0
-------
Solution 3)
Number of H3O+ = 3 ; HA = 4 , and A- = 3
Ka = 3 x 3/4 = 2.25
------
Solution 4
Number of H3O+ = 1; HA = 4, and A- = 1
Ka = 1 x 1/4 = 0.25
--------------
So,
Answer:
Solution 1 = 3
Solution 2 = 1
Solution 3 = 2
Solution 4 = 4