Step 1 - Understanding the relation between temperature and volume for a gas
There are three important variables that can modify the state of a gas: pressure, temperature and volume. They're all related by the ideal gas equation.
When pressure is kept constant, we can simplify this relation: temperature and volume become proportional, i.e., the greater the temperature, the greater the volume. This can be stated mathematically as:
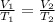
Step 2 - Substituting the values to solve the problem
From the exercise, we know that:
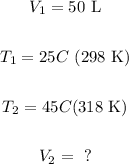
Note that we have converted all temperatures to Kelvin. That's because the proportionality between volume and temperature only work in K, the absolute temperature.
Substituting the values on the equation:
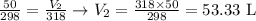
The final volume will be thus 53.33 L.