Answer
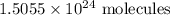
Step-by-step explanation
We need a conversion factor that will converts from moles of glucose, C6H12O6 to molecules of glucose.
That is, one mole of any substance contains 6.022 x 10²³ molecule of that substance.
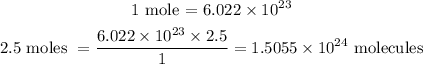
1.5055 x 10^24 molecules of glucose are in 2.5 moles of glucose C6H12O6