Answer : The number of moles of
 is 1.41 moles.
is 1.41 moles.
Explanation :
In
 , there are 2 nitrogen atoms and 3 oxygen atoms.
, there are 2 nitrogen atoms and 3 oxygen atoms.
As we know that, 1 mole of substance contains
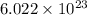 number of atoms.
number of atoms.
As,
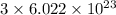 number of oxygen atoms contains 1 mole of
number of oxygen atoms contains 1 mole of

So,
 number of oxygen atoms contains
number of oxygen atoms contains
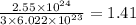 mole of
mole of

Therefore, the number of moles of
 is 1.41 moles.
is 1.41 moles.