The question is incomplete. The complete question is :
Calculate the wavelength of the electromagnetic radiation required to excite an electron from the ground state to the level with n = 6 in a one-dimensional box 34.0 pm in length.
Solution :
In an one dimensional box, energy of a particle is given by :
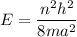
Here, h = Planck's constant
n = level of energy
= 6
m = mass of particle
a = box length
For n = 6, the energy associated is :
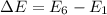
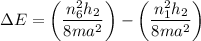
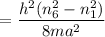
We know that,
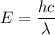
Here, λ = wavelength
h = Plank's constant
c = velocity of light
So the wavelength,
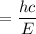
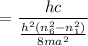
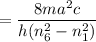
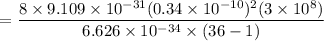
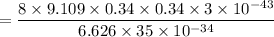
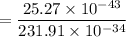
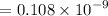 m
m
= 108 pm