30 KCal
Step-by-step explanationconstant we need

so
Step 1
To convert 300g ice -10° into ice at 0°
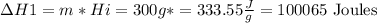
Step 2
to raise the temperature to 20° C
we need to use the fomrula
letQ = m•C•ΔT.
where m is the mass C is the
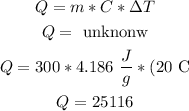
hence
the total heat need is
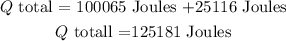
finally, to convert from Joules to Kcal , we need to multiply by 0.000239006
so
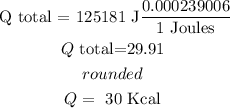
therefore, the answer is
30 KCal
I hope this helps you