To estimate the deltaH for the reaction we can do it from the bond energies, that is, starting from the energy needed to form or break a bond.
The reaction energy will be equal to the binding energy of the reactants minus the binding energy of the products. To calculate each bond energy we must first identify which bonds are involved in the reaction. They give us a balanced reaction:
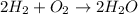
For the reaction, we have the following bonds
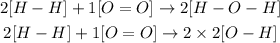
Now we are going to calculate the energy taking into account the values that we can find in tables of the bond energy.
Bond Ee (kJ/mol)
H - H 436
O=O 499
O - H 460
Therefore the energy on each side of the reaction will be:
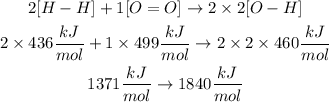
The reaction energy will be:
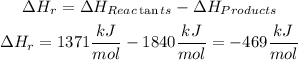
We have an exothermic reaction since the result is negative. This means that the energy of the products is greater than that of the reactants.
Now we will convert the energy units to kcal:
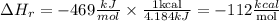
ΔH (kcal/mol) estimated of the reaction will be -112kcal/mol