Balanced equation:
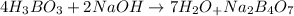
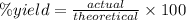
We need to determine theoretically how many grams of sodium tetraborate can be produced from 45g of NaOH.
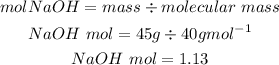
Based on the mole ratio, 2 moles of NaOH produces 1 mole of sodium tetraborate.

Theoretical value of sodium tetraborate is 217.38
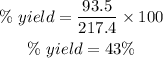
% yield is 43%