ANSWER
The final volume of the gas is 0.29L
Step-by-step explanation
Given that
The initial volume of the gas is 1 liters
The initial pressure of the gas is 25mmHg
The final pressure of the gas is 85mmHg
Follow the steps below to find the final volume of the gas
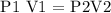

Therefore, the final volume of the gas is 0.29L