To solve this exercise, we are going to use Graham's Law of diffusion:
![\frac{rate1}{\text{rate}2}=\sqrt[]{(M2)/(M1)}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/4xoqs8hffd5ooi8skcks.png)
1 is for the rate and molar mass (M) of the first gas.
2 is for the second gas.
Data:
CO2 will be gas number 1
M1 = 44 g/mol
rate 1
-------------------------------------------------------------------------
Unknown gas is number 2
rate 2 and M2
rate 1 = 3.2 x rate 2
-------------------------------------------------------------------------
From Graham's law we clear M2:
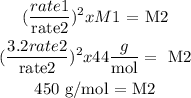
Answer: 450 g/mol