1) List the known and unknown quantities.
Volume: 347 mL.
Pressure: 6700 kPa.
Temperature: 27 ºC.
Ideal gas constant: 8.314 L * kPa * K^(-1) * mol^(-1).
Moles: unknown.
2) Set the equation.
Ideal gas equation

3) Convert units.
3.1- Convert the volume.
1L = 1000 mL
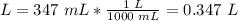
3.2- Convert the temperature
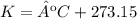
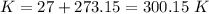
4) Plug in the known quantities (ideal gas equation) and solve for n (moles).
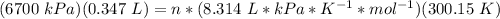
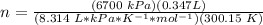
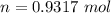
There would be 0.9317 mol N2.