Answer: "In the reaction N2(g) + 3H2(g) → 2NH3(g), nitrogen is reduced" (first option)
Step-by-step explanation:
The question requires us to choose, among the options given, which one best represents nitrogen gas (N2) in the reaction with hydrogen (H2) to produce ammonia (NH3).
The question provided the balanced chemical equation for the reaction:
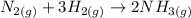
Analyzing the type of reaction, we can say that nitrogen was not synthesized, as N2 is a reactant in the process and not the product. In fact, NH3 was synthesized in this reaction.
Also, we can say that nitrogen was not electrolyzed, since there isn't a clear electrolysis process shown by the reaction.
To analyze if N2 was either oxidized or reduced, we must check the oxidation number of all species in the reaction:
- N in N2 has oxidation number 0 (because it is a homonuclear diatomic molecule);
- H in H2 has oxidation number 0 (because it is a homonuclear diatomic molecule);
- N in NH3 has oxidation number -3;
- H in NH3 has oxidation number +1.
From the oxidation numbers listed above, we can see that H suffered oxidation (oxidation number changed from 0 to +1) and N suffered reduction (oxidation number changed from 0 to -3).
Therefore, we can complete the sentence presented by the question as:
"In the reaction N2(g) + 3H2(g) → 2NH3(g), nitrogen is reduced" (first option)