Answer
359.84 grams
Step-by-step explanation
Given:
Number of molecules of sodium nitrate = 2.55 x 10²⁴ molecules
What to find:
The grams of sodium nitrate in 2.55 x 10²⁴ molecules.
Solution:
The first step is to convert 2.55 x 10²⁴ molecules of sodium nitrate to moles.
1 mole of any substance contains 6.023 × 10²³ molecules
Therefore 2.55 x 10²⁴ molecules of sodium nitrate is
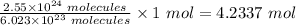
The final step is to convert the moles to mass using the mole formula.
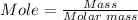
Molar mass of sodium nitrate = 84.9947 g/mol
Plugging mole = 4.2337 mol and molar mass = 84.9947 g/mol, the mass of the nitrate will be:
Mass = mole x molar mass = 4.2337 mol x 84.9947 g/mol
Mass = 359.84 grams.
Hence, 2.55 x 10²⁴ molecules of sodium nitrate would weigh 359.84 grams