The equation presented to us is balanced since we have the same number of atoms of each element on each side of the reaction. So we can continue with the calculations.
To determine the amount of product that we can obtain we must see the stoichiometry of the reaction. We will follow the following steps:
1. We find the moles of C6H6 contained in 15.7g by dividing the mass by the molar mass of C6H6 (78.11g/mol).
2. We find the moles of C6H5Cl that are produced using the ratio C6H5Cl to C6H6 which is equal to 1/1. This is because the coefficients that accompany these molecules are 1 for both.
3. We find the grams of C6H5Cl by multiplying the moles by the molar mass of C6H5Cl(112.56g/mol).
Let's proceed to do the calculations.
1. Moles of C6H6:
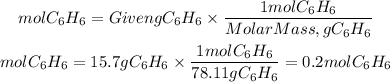
2. Moles of C6H5Cl
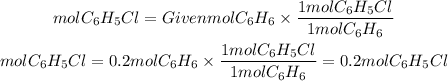
3. grams of C6H5Cl
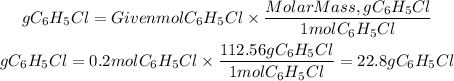
If we start with 15.7 of C6H6 we will obtain 22.8g C6H5Cl. The answer will be the first option: 22.8g