Answer:
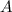
Step-by-step explanation:
Here, we want to know why the resulting compound is held together
The compound is formed by the combination of the sodium ion and bromide ion
While the sodium ion is positively charged, the bromide ion is negatively charged
These two come together to form the sodium bromide ion as a result of oppositely charged ions attracting each other