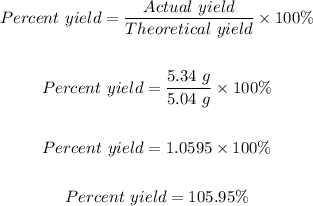
Answer
105.95%
Step-by-step explanation
Given:
Mass of Na = 3.74 g
Mass of Na2O recovered (actual yield) = 5.34 g
What to find:
The percent yield for the reaction
Step-by-step solution:
The first step is to write a balanced chemical equation for the reaction.
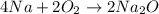
The next step is to calculate the theoretical yield of Na2O.
From the Periodic Table:
Molar mass of Na = 22.99 g/mol
Molar mass of Na2O = 61.98 g/mol
From the equation above, 4 moles of Na produced 2 moles of Na2O
In grams, 4 x 22.99 = 91.96 g of Na produced x 61.98 = 123.96 g of Na2O
So 3.74 g of Na will produce:
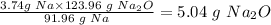
The theoretical yield of Na2O = 5.04 g
Therefore, the percent yield can of the reaction can be calculated as follows: