Answer:
 atoms
atoms
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number
 of particles.
of particles.
General representation of an element is given as:


where,
Z represents Atomic number
A represents Mass number
X represents the symbol of an element
1 mole of bromine
 contains=
contains=
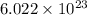 atoms
atoms
1.54 moles of bromine
 contains=
contains=
 atoms
atoms
Thus
 atoms are present in 1.54 moles of bromine.
atoms are present in 1.54 moles of bromine.