The Percentage Yield is 63.9%
Hello
To solve this problem, we would calculate the theoritical yield of the reaction first.
Theoritical Yield
The theoritical yield of the reaction can be calculated using the equation of reaction.
Given that 2 moles of C₂H₂ will produce 4 moles of CO₂
This implies that
2*26g (from molar mass of C₂H₂) will produce 4*44g of CO₂
52g of C₂H₂ = 176g of CO₂
21.5g of C₂H₂ = xg of CO₂
cross multiply and solve for x
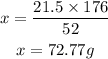
From the calculation above, the theoritical yield of CO₂ is 72.77g
Let's use this information and solve for the percentage yield.
Percentage Yield
This is the ratio between the actual yield to the theoritical yield and multiplied by 100.
Mathematically,
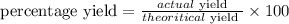
Data;
Actual yield = 46.5g
Theoritical yield = 72.77g
percentage yield = ?
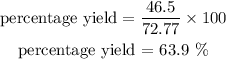
From the calculations above, the Percentage Yield is 63.9%