ANSWER
The number of moles of KNO3 is 0.539 moles
Explanation:
Given information
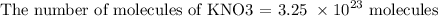
The next step is to find the number of moles by using the below number
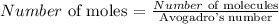
Recall that, Avogadro's number is 6.022 x 10^23

Therefore, the number of moles of KNO3 is 0.539 moles