1) Balancing a chemical equation. We have to balance a chemical equation in order to fulfill the requirement of the conservation of mass law. In a chemical reaction, the molecules of the reactants collide to each other and form new substances called products. For example,
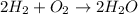
We know a chemical equation is balanced when the number of atoms of every element in the chemical reaction in the reactants side is the same number of atoms in the products.
B. The total number of atoms in the reactants must equal the number of total atoms in the products.