Answer: C)
 photons
photons
Step-by-step explanation:
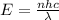
E= energy = 609 kJ = 609000 J (1kJ=1000J)
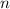 = number of photons = ?
= number of photons = ?
h = Planck's constant =
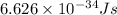
c = speed of light =
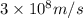
 = wavelength of photon = 589 nm =
= wavelength of photon = 589 nm =
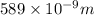
Putting values in above equation, we get:
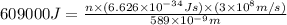
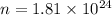
Thus there are
 photons in a burst of yellow light (589 nm) from a sodium lamp that contains 609 kJ of energy.
photons in a burst of yellow light (589 nm) from a sodium lamp that contains 609 kJ of energy.