ANSWER
the empirical formula is MgH8O3
Step-by-step explanation
Given that;
The % composition of Mg is 46.2%
The % composition of H is 7.69%
The % composition composition of O is 46.2%
Assume the mass of the sample is 100g
To find the empirical formula, follow the steps below
Step 1; Find the mass of the elements
For Magnesium
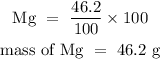
For hydrogen
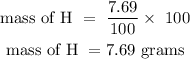
For oxygen
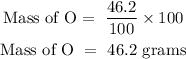
Step 2; Find the molar mass of the elements
The molar mass of oxygen is 15.999 g/mol
The molar mass of hydrogen is 1.00 g/mol
The molar mass of magnesium is 21.904.305 g/mol
Find the mole of the element
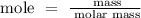
For Mg
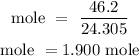
For Hydrogen
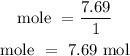
For Oxygen
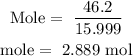
Step 3; find the mole ratio
In the above calculations, Mg has the least number of moles. Therefore to find the mole ratio divide the moles by the smallest moles
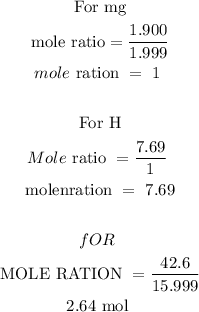
Therefore, the empirical formula is MgH8O3