Answer:
The balanced chemical equation is:
![2Al_2O_3\operatorname{\rightarrow}4Al+3O_2]()
- Ratio of Al2O3 to O2: 2:3R
- Ratio of Al2O3 to Al: 2:4R
- Ratio of Al to O2: 4:3R
Step-by-step explanation:
To balance the chemical equation, it is necessary to have the same amount of elements on the reactant side as on the product side:
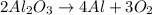
Now we know that the reaction is balanced, because on the reactant side and on the products side there are:
- 4 Al
- 6 O
Now that the equation is balanced, we can write the ratios with the stoichiometry of the reaction.